Atomic Theory
Atom, Electron & Electricity:
Everything starts from an atom and all objects on earth are made up of atoms. An atom is the basic building block of all matter. Figure in the right depicts classical structure of an atom. Before you can study electric and electronic devices, you need to have a solid understanding of Atoms and Electrons, andtheir bonding with each other.Once through with the concept, you will get to know what produces electricity and then understand the differences between conductors, insulators and semi-conductors. Let us start with an atom and its structure.
If you are interested in knowing only a brief overview on atomic theory, jump directly to “Conclusion”.
Atom as discussed is the basic building block of all objects in our known universe. If you say your house is made up of bricks and cement, your toy is made of plastic, your robot is made of electronic components; then each of those objects are made up of atoms. Atoms consist of a central nucleus and electrons revolving round it.Physicists across the worldhave proposed different model/structure of an atom. Till the end of 19th century, Atom was considered just a solid sphere like particle. Nobody knew, or the technology at that time was not able to answer the structure and contents of an Atom. The revolution happenedwhen Rutherford performed a series of experiments and fired tiny radioactive particles against objects like gold foil. The results, although not completely right, provided an insight on structure of an atom. Although he was not the first to conceptualize this, his tests confirmed their structure.
Rutherford Model
In 1911 Rutherfordproposed his view of an atom which contains a small dense positive 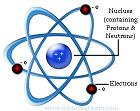 charge surrounded by negatively charged electron cloud where electrons revolve around the positive charge just like planets orbit the sun based on earlier atomic models. The central positive charge was later known as the nucleus. The figure in the right gives a conceptual view of his model.
charge surrounded by negatively charged electron cloud where electrons revolve around the positive charge just like planets orbit the sun based on earlier atomic models. The central positive charge was later known as the nucleus. The figure in the right gives a conceptual view of his model.
This Planetary model of atom had its limitations. According to classical physics when an electron (or particle) is accelerating or decelerating, itemits electromagnetic radiation. In this case, electrons in Rutherford model radiate all energy and finally collapse inwards into the nucleus. For some unknown reason, this model is best visualized by most of us although it is an inaccurate portrayal of an atom structure.
In 1932, James Chadwick, an associate of Rutherford confirmed the existence of Neutrons. Although there were previous assumptions of existence of Neutron, James Chadwick proved its existence through a series of tests. Accordingly, there is a central nucleus which contains Protons and Neutrons, and electrons revolving around.
Niels Bohr Model
To overcome the limitations of Rutherford’s model, Niels Bohr, a Danish 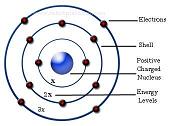 Physicist proposed a modified version in 1913. Electrons revolve around the nucleus in certain special orbits and each of these orbits has different energy levels associated known as energy shells (or energy level measured in electron volt). The orbit closest to the nucleus has the lowest energy level and the orbit farthest to the nucleus has the highest energy level. Since electrons move in a particular orbit, they do not lose energy. When an atom absorbs energy, these electrons get excited and jump to the next higher energy level. Once they radiate energy they drop down to a lower energy level. As a next level, he also said that each of those discrete orbits can contain only a certain number of maximum electrons and once the orbit is full, electrons occupy the next orbit with higher energy level. This model is well known and all recent models are based on this design. Again, this is the same simple model taught in most elementary physics due to its ease of understandability.
Physicist proposed a modified version in 1913. Electrons revolve around the nucleus in certain special orbits and each of these orbits has different energy levels associated known as energy shells (or energy level measured in electron volt). The orbit closest to the nucleus has the lowest energy level and the orbit farthest to the nucleus has the highest energy level. Since electrons move in a particular orbit, they do not lose energy. When an atom absorbs energy, these electrons get excited and jump to the next higher energy level. Once they radiate energy they drop down to a lower energy level. As a next level, he also said that each of those discrete orbits can contain only a certain number of maximum electrons and once the orbit is full, electrons occupy the next orbit with higher energy level. This model is well known and all recent models are based on this design. Again, this is the same simple model taught in most elementary physics due to its ease of understandability.
As expected, different experiments exposed different results and this model still did not answer to some basic physicist’s questions. Why should electrons occupy only certain energy levels with definite orbits with definite radius? If the electrons orbit in a circular motion, then they need to give off energy when they accelerate. Why do they radiate only on jumps from one energy level to another. There was something missing in this model. These questions were answered by Quantum Mechanics.
Do you have anything to say?
Visit the Forum to discuss, learn and share anything related to robotics and electronics !!








